Science
Researchers Uncover Secrets of Carbon’s Journey to Diamond
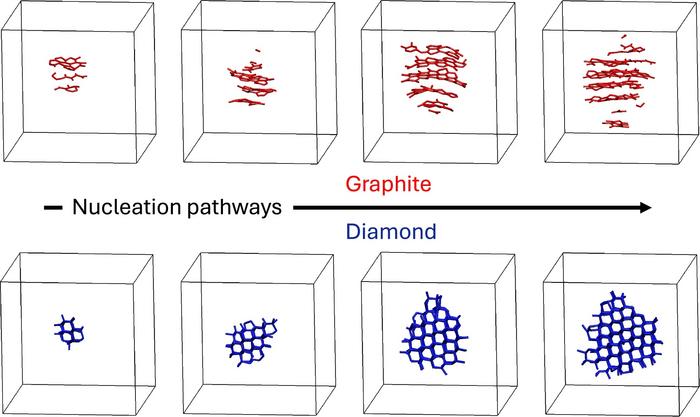
Recent research from the University of California, Davis, has unveiled new insights into how molten carbon crystallizes into either diamond or graphite, two distinct forms of carbon with vastly different properties. This study, led by chemist Davide Donadio, employs advanced simulations that leverage machine learning to predict molecular behavior, shedding light on processes crucial for various fields, from geology and nuclear fusion to quantum computing and industrial diamond production.
The ability to monitor the crystallization of molten carbon presents significant challenges due to the rapid nature of the process and the extreme conditions required. Traditional experiments that sought to replicate these conditions, particularly through high-pressure flash heating, have yielded inconsistent results. Understanding the phase changes during crystallization could greatly enhance the production of synthetic diamonds, nanodiamonds, and other carbon-based materials.
Simulations Reveal Complex Crystallization Behavior
In their work, Donadio and his team utilized machine-learning-accelerated, quantum-accurate molecular dynamics simulations to explore how diamond and graphite form as liquid carbon cools from 5,000 K to 3,000 K under pressures ranging from 5 to 30 GPa. While extreme conditions can be generated through techniques like laser heating, these methods require specialized equipment. The simulations provide a controlled environment, allowing for detailed observation of the crystallization process at the atomic level.
The team’s findings indicate that the crystallization behavior of molten carbon is more intricate than previously understood. While diamond forms under higher pressures, graphite emerges at lower pressures, even though diamond is thermodynamically more stable in those conditions. This unexpected behavior can be attributed to Ostwald’s step rule, which posits that crystallization often occurs through intermediate metastable phases rather than directly transitioning to the most stable form. In this case, the structure of graphite closely resembles that of the liquid carbon, making it easier for the liquid to crystallize into graphite first.
“The liquid carbon essentially finds it easier to become graphite first, even though diamond is ultimately more stable under these conditions,” explains Tianshu Li, a professor of civil and environmental engineering at George Washington University. “It’s nature taking the path of least resistance.”
Implications for Future Research and Applications
The insights from this research, published in Nature Communications, could help reconcile discrepancies in historical electrical and laser flash-heating experiments aimed at mapping carbon’s phase diagram. These earlier studies may have inadvertently trapped their systems in metastable graphitic configurations, hindering the understanding of the transition between phases.
Donadio has a rich background in crystal nucleation, having studied it for over two decades. He remarks, “Studies based on so-called empirical potentials have been typically unreliable in this context, while ab initio density functional theory-based calculations are often too slow. Machine learning potentials allow us to overcome these issues, offering the right combination of accuracy and computational speed.”
Looking ahead, Donadio and his colleagues plan to investigate more complex chemical compositions and focus on specific pressures and temperatures that resemble those found in the interiors of giant planets within our solar system. This research not only enhances the understanding of carbon but also opens avenues for practical applications in material science and technology.
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoMilk Bank Urges Mothers to Donate for Premature Babies’ Health
-

 Lifestyle2 weeks ago
Lifestyle2 weeks agoShoppers Flock to Discounted Neck Pillow on Amazon for Travel Comfort
-

 Politics3 weeks ago
Politics3 weeks agoMuseums Body Critiques EHRC Proposals on Gender Facilities
-

 Business3 weeks ago
Business3 weeks agoTrump Visits Europe: Business, Politics, or Leisure?
-

 Politics2 weeks ago
Politics2 weeks agoCouple Shares Inspiring Love Story Defying Height Stereotypes
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoJapanese Teen Sorato Shimizu Breaks U18 100m Record in 10 Seconds
-

 World3 weeks ago
World3 weeks agoAnglian Water Raises Concerns Over Proposed AI Data Centre
-

 Sports3 weeks ago
Sports3 weeks agoBournemouth Dominates Everton with 3-0 Victory in Premier League Summer Series
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoShoppers Rave About Roman’s £42 Midi Dress, Calling It ‘Elegant’
-

 World3 weeks ago
World3 weeks agoWreckage of Missing Russian Passenger Plane Discovered in Flames
-

 World3 weeks ago
World3 weeks agoInquest Resumes for Jay Slater Following Teen’s Tragic Death
-

 Sports3 weeks ago
Sports3 weeks agoSeaham Red Star Begins New Chapter After Relegation Setback









