Health
Experts Warn Against Unapproved Weight Loss Drug Sold Online
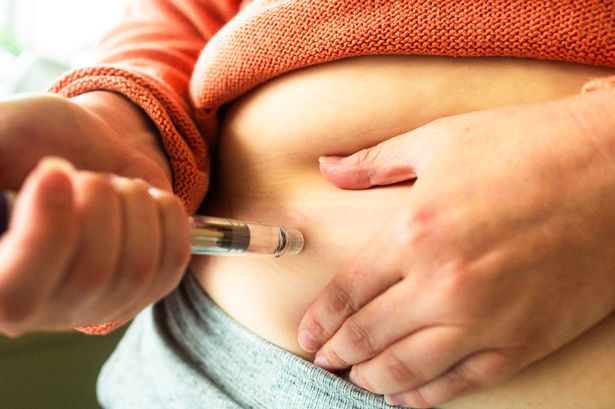
Medical professionals are raising alarms about an unapproved weight-loss drug known as Retatrutide, informally referred to as “Reta” or the “Godzilla of fat-jabs.” Despite not receiving regulatory approval, the drug is being sold online in the UK, posing significant health risks to potential users. As an experimental treatment currently undergoing clinical trials by its manufacturer, Eli Lilly, Retatrutide is not legally available for sale.
Reports indicate that various online vendors are offering vials of this drug, which are marketed for self-injection after mixing with sterile water. These shops are not registered pharmaceutical wholesalers, and their marketing strategies appear to directly target consumers. While some product listings include disclaimers like “For laboratory use only—not for human consumption,” there are no identity checks required for purchasing, thus making it accessible to the general public.
Concerns about safety have been echoed by a spokesperson from Oxford Online Pharmacy, who stated that the sale of such drugs encourages individuals lacking medical expertise to concoct their own weight-loss solutions and self-administer them without medical supervision. They emphasized that medications like Wegovy and Mounjaro, which are approved for weight loss, should only be used under the guidance of qualified healthcare professionals.
The risks associated with using unregulated drugs are substantial. The spokesperson warned, “If you see Retatrutide for sale online (not by a registered pharmacy) or on social media, you could be risking your life. There is no guarantee of the safety or efficacy of what you are putting into your body.”
Retatrutide aims to support weight loss through multiple mechanisms and has already garnered attention with its nicknames, including “Triple G.” It is designed to be administered via a pen injector, delivering subcutaneous injections into areas such as the arm, thigh, or abdomen. Expected to be given weekly, the treatment will start with low doses to mitigate side effects.
While clinical trials have shown promising results, the drug remains in the latter stages of testing (Phase 3), and its availability is not imminent. If all goes well, it could be accessible by 2027, but it will be classified as a prescription-only medication, meaning it cannot be purchased over-the-counter.
The regulatory approval process is rigorous. In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) must evaluate trial data before any drug can receive approval. Following this, for the drug to be distributed via the National Health Service (NHS) or its equivalents in the UK regions, it must receive endorsement from various governmental bodies, including:
– England: National Institute for Health and Care Excellence (NICE)
– Scotland: Scottish Medicines Consortium (SMC)
– Wales: All Wales Medicines Strategy Group (AWMSG)
– Northern Ireland: Department of Health, Social Services and Public Safety (DHSSPS)
This approval process can often take months or even years, underscoring the importance of caution when considering unregulated weight-loss drugs. With the increasing temptation of online purchases, the risks associated with unverified products become even clearer.
-

 Entertainment2 months ago
Entertainment2 months agoIconic 90s TV Show House Hits Market for £1.1 Million
-

 Lifestyle4 months ago
Lifestyle4 months agoMilk Bank Urges Mothers to Donate for Premature Babies’ Health
-

 Sports3 months ago
Sports3 months agoAlessia Russo Signs Long-Term Deal with Arsenal Ahead of WSL Season
-

 Lifestyle4 months ago
Lifestyle4 months agoShoppers Flock to Discounted Neck Pillow on Amazon for Travel Comfort
-

 Politics4 months ago
Politics4 months agoMuseums Body Critiques EHRC Proposals on Gender Facilities
-

 Business4 months ago
Business4 months agoTrump Visits Europe: Business, Politics, or Leisure?
-

 Lifestyle4 months ago
Lifestyle4 months agoJapanese Teen Sorato Shimizu Breaks U18 100m Record in 10 Seconds
-

 Politics4 months ago
Politics4 months agoCouple Shares Inspiring Love Story Defying Height Stereotypes
-

 World4 months ago
World4 months agoAnglian Water Raises Concerns Over Proposed AI Data Centre
-

 Sports4 months ago
Sports4 months agoBournemouth Dominates Everton with 3-0 Victory in Premier League Summer Series
-

 World4 months ago
World4 months agoWreckage of Missing Russian Passenger Plane Discovered in Flames
-

 Lifestyle4 months ago
Lifestyle4 months agoShoppers Rave About Roman’s £42 Midi Dress, Calling It ‘Elegant’









